Mitochon Pipeline
MP101
MP201
Traumatic Brain Injury (TBI)
MP201 Significantly Protects Cortex From Blunt Trauma.
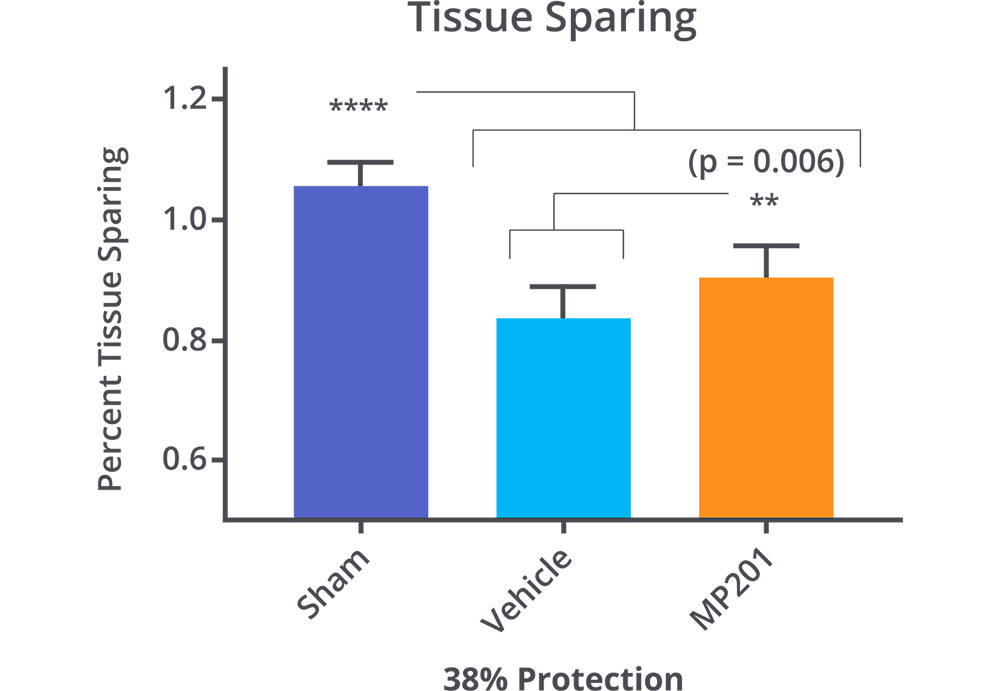
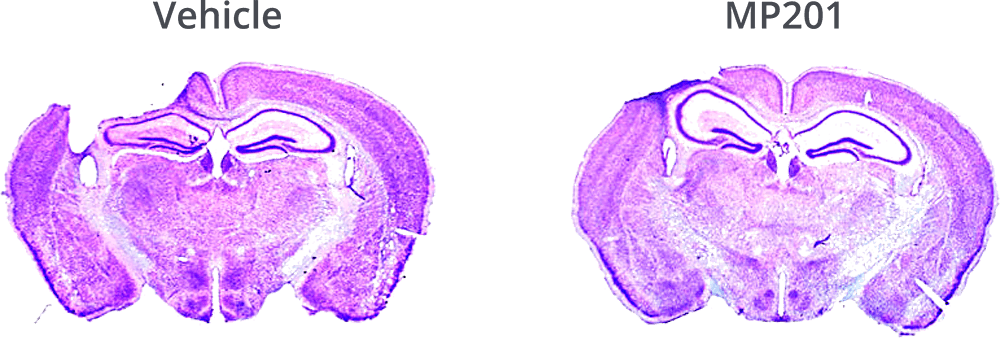
Hubbard, W.B., et al., Mitochondrial uncoupling prodrug improves tissue sparing, cognitive outcome, and mitochondrial bioenergetics after traumatic brain injury in male mice. J Neurosci Res, 2018.
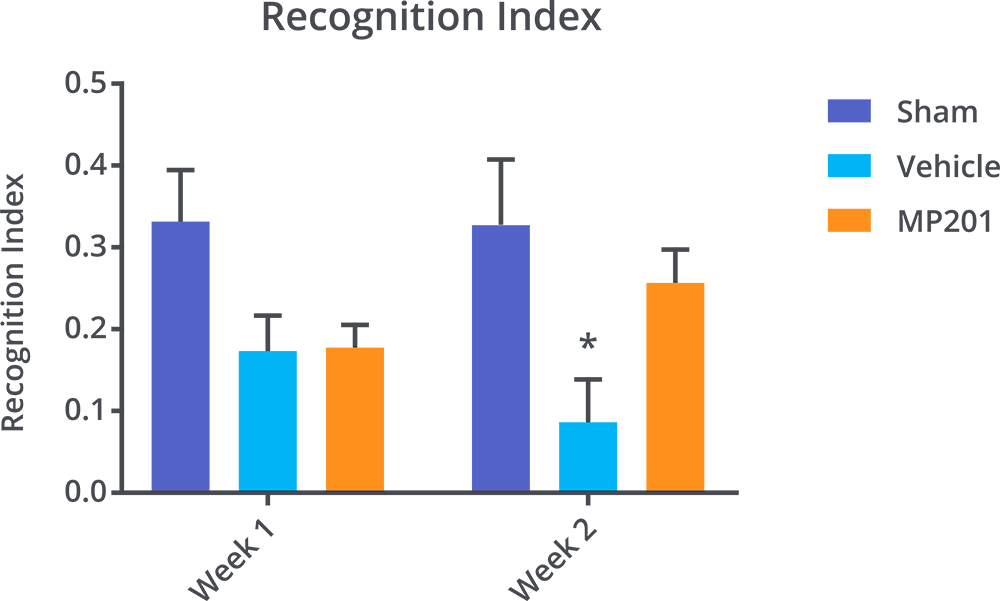
MP201 Leads To Cognitive Improvement Post TBI